Compliance for regulated industries now available!
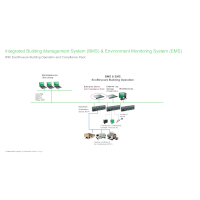
Now pursue opportunities in highly regulated pharma, biotech and medical device manufacturing industries! Compliance Pack for EcoStruxure Building Operation enables 21 CFR Part 11 compliance (and more), with required features including:
- Facility-wide accountability and traceability with electronic signatures and secure electronic records
- Easy search & retrieval with complete archives of system data and events in one reporting structure
- Ease of auditing with segregated or integrated building management and environmental monitoring
Find all materials for the Compliance Pack for Regulated Industries here.